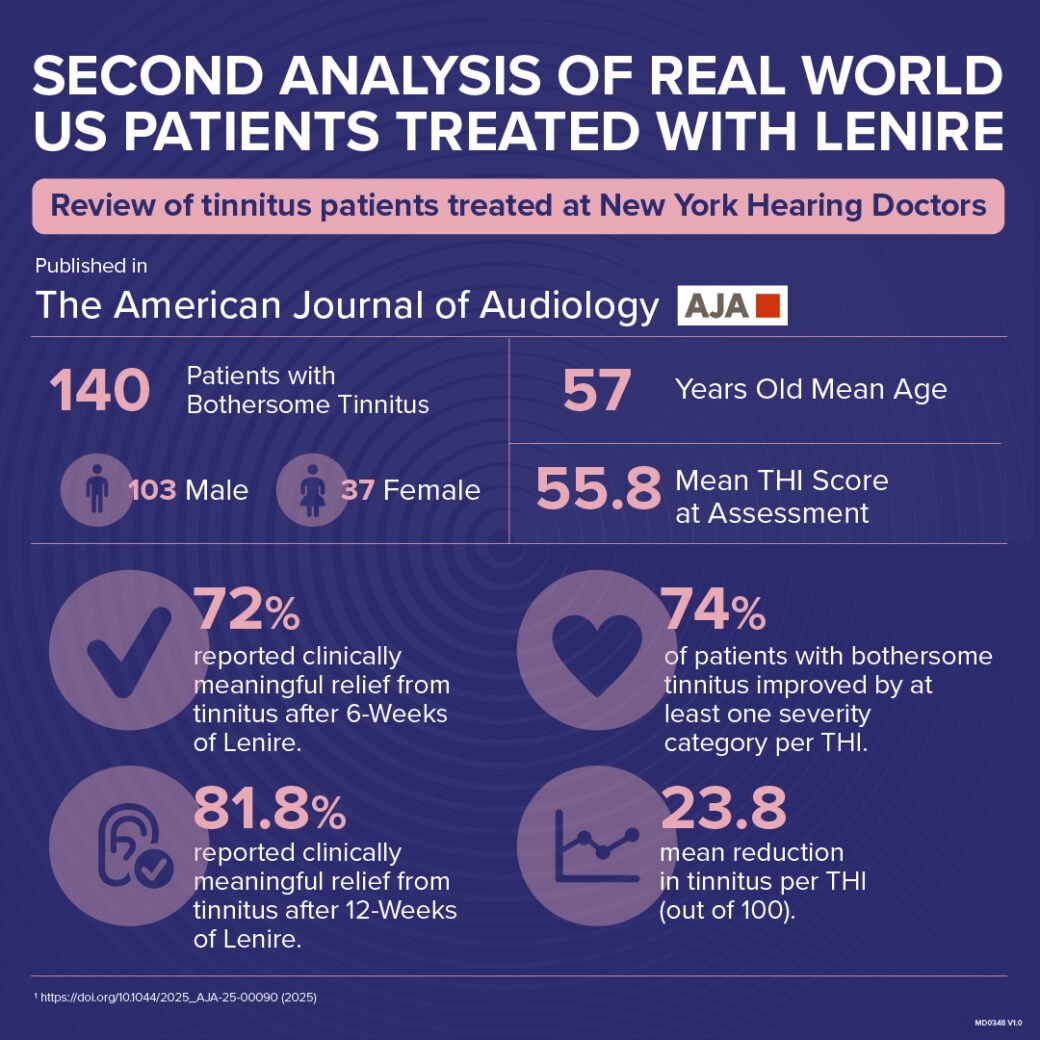
- 81.8% of patients with bothersome tinnitus treated with Lenire at New York Hearing Doctors reported significant improvement in a real-world clinical setting in Lenire real patient analysis.1,2
- Patients with bothersome tinnitus reported a 23.8 point mean reduction in tinnitus as measured by the Tinnitus Handicap Inventory, which is greater than three times the threshold for clinical significance.1,2
- The majority of patients with bothersome tinnitus reported they were no longer severely impacted by their tinnitus following 12-weeks of Lenire.1,2
- This evidence is consistent with the first real world analysis of U.S. tinnitus patients treated with Lenire that was peer-reviewed and published in Nature Communications Medicine.3
New York, February 17, 2025: The American Journal of Audiology has peer-reviewed and published the positive outcomes of U.S. tinnitus patients treated with Lenire, the only FDA approved tinnitus treatment device of its kind.
Results in the paper titled: “Bimodal Neuromodulation for Tinnitus in a Clinical Practice Setting: Clinically Significant Benefit for Patients with Moderate or Worse Symptoms” reported 81.8% of patients with bothersome tinnitus had a clinically significant reduction in tinnitus when treated with Lenire.1,2
- Read the paper: https://pubs.asha.org/doi/10.1044/2025_AJA-25-00090
Largest Body of Real World Evidence for a Bimodal Tinnitus Treatment Device
These Lenire real patient anlaysis results are consistent with previously published Lenire treatment outcomes. This shows repeatable success for tinnitus patients in real world clinical settings.
In addition to being supported by the largest body of clinical trial data in its field, Lenire is now underpinned by the most extensive real-world evidence of safety and effectiveness, following publication of this research in The American Journal of Audiology.
The paper, authored by Dr. Craig Kasper Au.D of New York Hearing Doctors (NYHD) et al, analyzed the results of 140 tinnitus patients who were fitted with Lenire at NYHD between May 1, 2023, and January 19, 2024.
Tinnitus is commonly known as ringing in the ears but can manifest as hissing, buzzing, and other persistent sounds. The condition afflicts an estimated 25 million American adults4, with an estimated 2.5 million tinnitus patients living in New York alone.5
American Journal of Audiology Publishes Lenire Real Patient Analysis
Lenire uses bimodal neuromodulation to treat tinnitus. Bimodal neuromodulation is the simultaneous stimulation of two nerves for therapeutic purposes. Lenire plays audio tones via headphones while delivering mild energy pulses to the surface of the tongue to treat tinnitus. Under the care of an audiologist with tinnitus expertise, patients with bothersome tinnitus typically use the device at home for two 30-minute sessions daily for approximately 12 weeks.
This paper is the second in a series of planned, real-world evidence publications that have been compiled from thousands of U.S. tinnitus patients that have been successfully treated with Lenire.
The analysis found that at the interim check-up, after six weeks of treatment with Lenire, 72.6 percent of patients with bothersome tinnitus had a clinically meaningful reduction in tinnitus.1,2 After 12-weeks, 81.8% of patients with bothersome tinnitus had a clinically meaningful reduction.1,2
Patients reported a mean reduction of 23.8 points on the Tinnitus Handicap Inventory (THI) after 12-weeks, greater than three times the threshold for clinically significant reduction.1,2 As a result, the majority of patients with bothersome tinnitus reported they were no longer severely impacted by their tinnitus following 12-weeks of Lenire, according to tinnitus severity grading guidelines.1, 2, 11

Lenire is“Nothing Less Than a Game-Changer”
“New York Hearing Doctors stay on the cutting-edge of tinnitus care through the introduction of modern technologies like Lenire and leveraging research to consistently refine our treatment methodologies,” said NYHD founder, Dr. Craig Kasper, Au. D. “The combination of our personalized approach to tinnitus care and the remarkable effectiveness of Lenire, we are seeing life-changing treatment outcomes for our patients.”
“Lenire was nothing less than a game-changer in my life. I went from debilitating, almost catastrophic tinnitus, that required medication to treat the depression and anxiety, to being able to enjoy life again after four months of Lenire.” said Richard Bistrong, tinnitus patient at New York Hearing Doctors, “Three years later, I can enjoy my life and not worry about my tinnitus. This has impacted not only my well-being but my loved ones as well. For anyone that is looking for relief, that is based in science and patient results, I would encourage you to seek your local Lenire Provider as soon as possible to learn more about Lenire.”
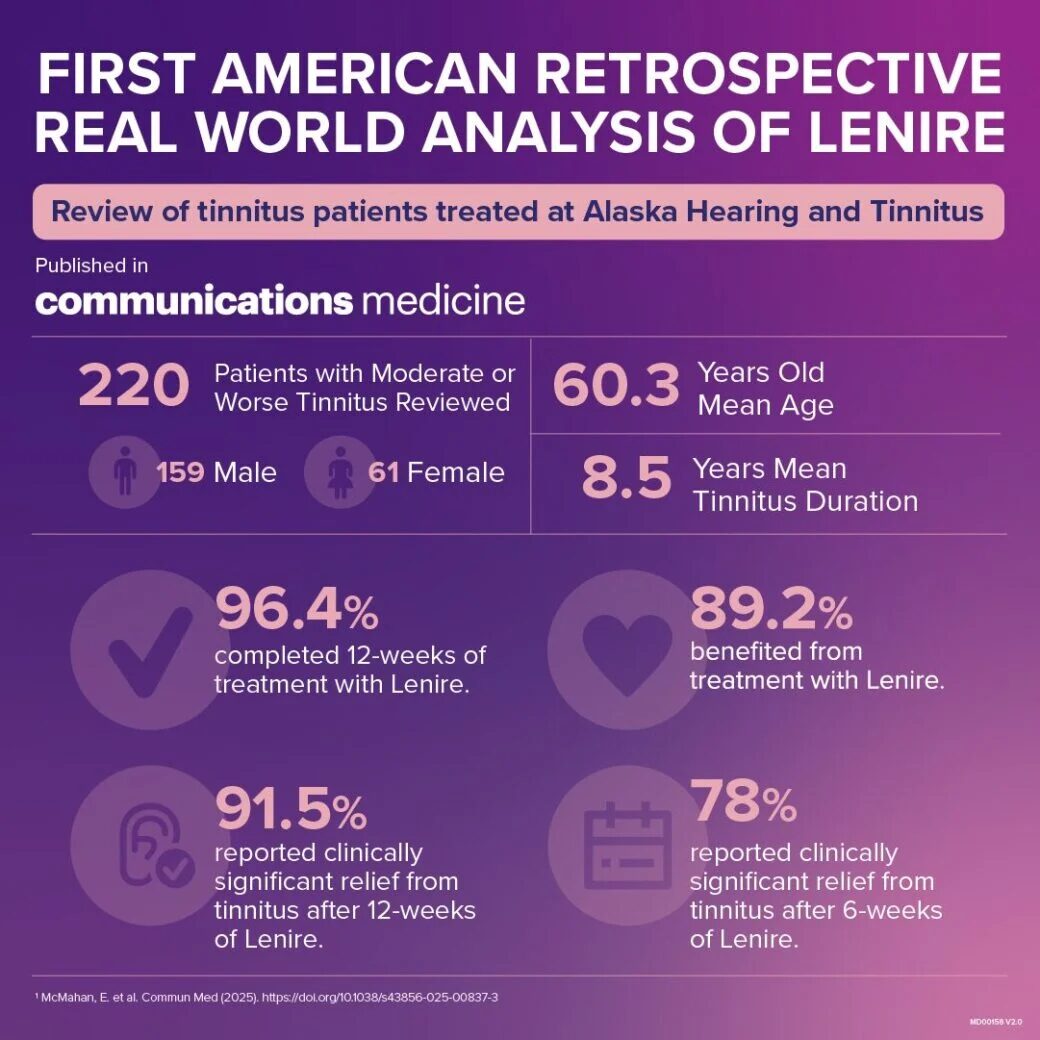
Data Summary from Lenire’s 1st Published RWE Paper.
Lenire Real Patient Analysis Consistent with 1st Peer-Reviewed and Published Paper
The evidence in this paper is consistent with the first peer-reviewed real-world analysis of U.S. tinnitus patients treated with Lenire. It is also consistent with results from Lenire’s large-scale clinical trials.3,6,7,8
The first real world analysis of U.S. patients treated with Lenire was peer-reviewed and published in Nature Communications Medicine. It showed that 91.5% of Lenire patients had a clinically significant reduction in tinnitus after 12-weeks. This consistency demonstrates the effectiveness of a typical treatment protocol with Lenire in a real-world clinical setting.
Lenire’s controlled TENT-A3 clinical trial facilitated FDA approval in March 2023, and Nature Communications published the results. The paper remains in the 99th percentile of more than 250,000 tracked Nature articles.
TENT-A3 included 112 trial participants. The trial demonstrated Lenire’s clinical superiority to sound-only therapy, a widely used treatment for tinnitus. Nearly 89% of trial participants said they would recommend Lenire as a tinnitus treatment.6
According to Neuromod Devices founder and CEO, Dr. Ross O’Neill, Lenire’s principal inventor: “The consistency of the real-world outcomes of US tinnitus patients treated with Lenire with our large-scale clinical trials demonstrates the replicability and scalability of Lenire as a tinnitus treatment option for the over 740 million people worldwide living with tinnitus.”
“By working closely with our network of providers, we are seeing market-surpassing patient outcomes, improving clinical best practices, and a rapidly growing body of robust real-world evidence positioning Lenire and bimodal neuromodulation as a leading tinnitus treatment option.”
Lenire is available through specialized tinnitus clinics in the United States of America and Europe. Lenire is also a treatment option through the US Department of Veterans Affairs.
References and Notes
- Kasper, C et al. Bimodal Neuromodulation for Tinnitus in a Clinical Practice Setting: Clinically Significant Benefit for Patients with Moderate or Worse Symptoms, American Journal of Audiology, https://doi.org/10.1044/2025_AJA-25-00090 (2025)
- As measured by Tinnitus Handicap Inventory (THI).
- Mc Mahan, E., and Lim, H. Retrospective chart review demonstrating effectiveness of bimodal neuromodulation for tinnitus treatment in a clinical setting Commun Med (2025). https://doi.org/10.1038/s43856-025-00837-3
- https://www.nidcd.nih.gov/health/tinnitus
- https://www.nyc.gov/assets/doh/downloads/pdf/survey/survey-2013noise.pdf
- Boedts, M. Beuchner, A. et al. Combining sound with tongue stimulation for the treatment of tinnitus: a multi-site single-arm controlled pivotal trial. Nature communications (2024)
- Conlon et al., Sci. Transl. Med. 12, eabb2830 (2020)
- Conlon et al., Different bimodal neuromodulation settings reduce tinnitus symptoms in a large randomized trial, Sci Rep.
- US VA Benefits Report Fiscal Year 2024: https://www.benefits.va.gov/REPORTS/abr/
- Zeman, F. et al, Tinnitus handicap inventory for evaluating treatment effects: which changes are clinically relevant? https://pubmed.ncbi.nlm.nih.gov/21493265/ (2011).
- A. McCombe et al., Guidelines for the grading of tinnitus severity, Clin. Otolaryngol. 2001, 26, 388 – 393
About Neuromod
Founded in 2010, Neuromod Devices is a global medical technology company. Neuromod has offices in Ireland, and the United States of America. The company specializes in the design and development of neuromodulation technologies. These technologies address the clinical needs of underserved patient populations who live with chronic and debilitating conditions.
The lead application of Neuromod’s technology is in the field of tinnitus. Neuromod has completed extensive clinical trials to confirm the efficacy of its non-invasive neuromodulation platform in this common disorder. For more information visit www.neuromoddevices.com.
About Lenire®
Lenire® is the first non-invasive bimodal neuromodulation tinnitus treatment device. The device has demonstrated its ability to soothe and relieve tinnitus in large-scale clinical trials.
Bimodal neuromodulation stimulates nerves using two paired stimuli for therapeutic purposes. Neuromod Devices developed Lenire, the tinnitus treatment device used in the study. Lenire consists of wireless (Bluetooth ®) headphones that deliver sequences of audio tones to both ears. The device delivers electrical stimulation pulses to the surface of the tongue through 32 electrodes, which are part of a proprietary device trademarked as Tonguetip®. Clinicians can configure the device’s settings to deliver treatment using different combinations of audio and electrical stimuli.
An easy-to-use handheld controller controls the timing, intensity, and delivery of the stimuli. Clinicians train each participant to use it before continuing treatment at home. Appropriately qualified healthcare professionals, such as ENTs or audiologists, prescribe Lenire after conducting a suitability assessment. Patients then complete treatment from the comfort of their own home.
Lenire has the largest volume of clinical trial and real world evidence for a bimodal neuromodulation device.
Lenire® has CE-mark certification for the treatment of tinnitus under the supervision of an appropriately qualified healthcare professional in Europe. The US FDA awarded Lenire a De Novo Approval Grant.
About Tinnitus
Tinnitus, known as ‘ringing in the ears,’ is a neurological condition where a person perceives sound without an external source. Researchers estimate that at least 25 million Americans currently live with tinnitus. The United States Veterans Administration (VA) compensates tinnitus as its most prevalent service-connected disability. More than 3.2 million veterans compensated in 2024.⁹
About Dr. Craig Kasper Au. D.
Dr. Craig Kasper, Au. D. is the founder and managing director of audiology clinic, New York Hearing Doctors & Institute for Hearing and Balance. He is also the owner and found of New York Hearing Doctors | Tinnitus Care, based in New York.
He earned his Doctorate of Audiology from the University of Florida. Dr. Kasper also holds a master’s degree with clinical honors from the State University of New York at Buffalo.
Dr. Kasper’s extensive experience includes a Clinical Fellowship and clinical practice. This is held in the Department of Otolaryngology/Head & Neck Surgery at New York-Presbyterian Medical Center. Dr. Kasper is a Distinguished Fellow of the National Academies of Practice. He is also a Fellow of the American Academy of Audiology (AAA), Dr. Kasper is a member of the Academy of Doctors of Audiology (ADA).
Dr. Kasper’s contributions extend beyond clinical practice; he has authored scholarly articles published in prestigious peer-reviewed journals such as Hearing Research and Laryngoscope and has been an invited presenter for professional conferences since the start of his career. Committed to public health education, he has also frequently served as a resource for the popular media on topics related to hearing health and wellness.