Lenire Tinnitus Treatment Device
Lenire is an at-home FDA approved tinnitus treatment device proven in clinical trials and with real world patients.


91% of 191 clinical trial patients treated with Lenire had a reduction in tinnitus that sustained for at least 12-months.
91.5% of 220 real world tinnitus patients treated with Lenire had a clinically significant reduction in tinnitus.
83% of 500+ patients asked across three large-scale clinical trials would recommend Lenire to treat tinnitus.
What is Lenire?
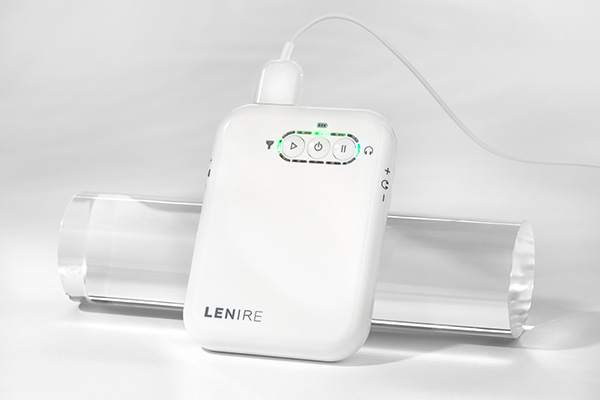
A handheld device used to adjust the timing, audio volume and tongue stimulation intensity during Lenire treatment.

A proprietary peripheral with 32-electrodes that sends mild pulses to the surface of the tongue.

Plays audio that delivers auditory stimulation synchronised with the Lenire Tonguetip.
Safe and Effective Tinnitus Treatment Device
Watch this explainer video to learn about how the Lenire Tinnitus Treatment Device works to soothe tinnitus.
Next Generation Tinnitus Treatment Technology
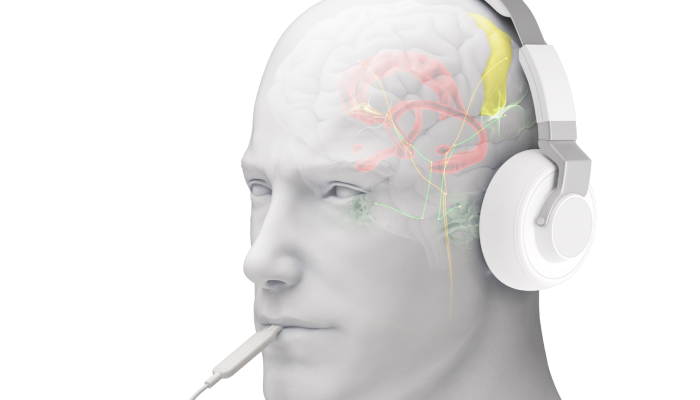
Neuromodulation is targeted stimulus delivery used to alter nerve activity. Bimodal neuromodulation stimulates two points of the nervous system at once. Lenire leverages this technology to soothe tinnitus.

Tinnitus Affects 15% of the Global Adult Population

Tinnitus, commonly known as ringing, hissing, or buzzing in the ears, affects millions of people around the world.

Proven in Three Large Scale Clinical Trials

80% of Lenire’s first clinical trial patients had a reduction in tinnitus severity that sustained for at least 12 months after treatment. 1

91% of Lenire’s second clinical trial patients had a reduction in tinnitus severity that sustained for at least 12 months after treatment. 2
Majority of patients with moderate or worse tinnitus had significant relief using Lenire when sound-only, the trial’s control, had no meaningful impact.3
Find a Lenire Clinic
Available in more than 130 clinics across the United States of America and Europe. Book an appointment with a leading tinnitus expert and start your tinnitus treatment journey.
Find a Lenire Provider
Frequently Asked Questions
Lenire is a bimodal neuromodulation tinnitus treatment device. The device combines mild pulses to the tongue with audio stimulation through headphones to retrain the brain to ignore tinnitus. The tinnitus treatment device has been proven safe and effective in three-large scale clinical trials involving 600+ patients.
Available in the United States of America and Europe. Lenire is exclusively accessible through suitably qualified and trained hearing healthcare professionals that specialise in tinnitus. Visit www.lenire.com/find-a-clinic to find a clinic near you.
Bimodal neuromodulation is the stimulation of two points of the nervous system at once. Bimodal neuromodulation in tinnitus has been proven as an effective way of retraining the brain’s response to tinnitus.
De Novo FDA is a FDA Approval pathway used for novel medical devices such as Lenire. Many medical devices use 510k Clearance. 510k relies on predicate, or an iteration on an existing approved device. De Novo FDA Grants require a medical device company to establish an entirely new device category and submit independent clinical data from clinical trials. Sometimes these trials must be supplemented with real-world evidence for the FDA experts to review. Lenire is the first and only tinnitus treatment device to receive De Novo approval from the FDA.
The device is used at home for two 30-minute sessions per day for a period of time recommended by a patient’s clinician.